TAMİFLU’ YA HARCANAN MİLYARLAR ÇÖPE Mİ GİTTİ?
Dikkat: Yazının sonunda ek var!
***
Dünyanın en muteber tıp dergilerinden olan BMJ’ nin (British Medical Journal) baş editörlerinden Dr. Fiona Godlee, ilaç devi Roche’ dan üç sene önce verdiği sözü tutarak Tamiflu ile ilgili tüm klinik araştırma raporlarının bağımsız araştırmalar için açıklanmasını istedi (1).
Şirket müdürü Profesör John Bell’ e açık bir mektup gönderen Godlee “Halkın milyarlarca lirası Tamiflu’ ya harcandı ama ilacın etkinliğini ve güvenilirliğini gösteren delillere ulaşılamıyor” diyor.
Godlee’ nin bu mektubu EMA’ nın (Avrupa İlaç Ajansı) Roche hakkında ilaç yan etkileriyle alâkalı 80 bin yan etkiyi uygun şekilde bildirmediği iddiasıyla ihlâl davası açmasından hemen sonra geldi.
Godlee, Times gazetesinde yayınlanan ‘ilaç firmalarını dürüst olmaya davet eden; araştırmacıların bugün kullanılmakta olan ilaçlarla alâkalı tüm klinik araştırma verilerine ulaşabilmelerinin sağlanmasını isteyen’ mektubun 28 imzacısı arasında da yer alıyor.
Politikacılardan da büyük baskılar geliyor. Geçen hafta Muhafazakâr Parti milletvekili Sarah Wollaston ‘eksik veri’ meselesini gündeme getirdi.
Sağlık Bakanı Norman Lamb da, klinik araştırma verilerine ulaşılabilmesi problemini tartışmak üzere uzmanları bir araya getirmeyi kabul etti.
Roche 3 sene önce söz vermişti
Roche firması, 2009 aralık ayında BMJ ve Cochrane Grubunun araştırmalarına cevap olarak Tamiflu ile ilgili tüm klinik raporları açıklayacağını îlânen vaat etmişti.
Bu araştırmada Tamiflu’ nun sağlıklı insanlarda zatürree gibi komplikasyonları önlediğine dair açık deliller olmadığı gösterilmişti.
Ayrıca da ilaç verilerine ulaşmayla, araştırmalarda hayalet yazar kullanılmasıyla ve ilacın onay süreciyle alâkalı olarak ciddi kaygılar olduğu bildirilmişti.
Bu araştırmadan sonra Cochrane uzmanlarına daha fazla bilgi verilmiş ama tüm veriler hiçbir zaman sağlanmamıştı.
Cochrane uzmanları, Tamiflu ile yapılmış en azından 123 çalışma olduğunu ve Roche’ nin tamamlanmış faz 3 çalışmalarının hasta verilerinin çoğunun (%60) hiç yayınlanmadığını söylüyor ve temel endişelerinin “ilacın etkinliğinin abartılması ve potansiyel ciddi yan etkilerinin küçümsenmesi” olduğunu ifade ediyorlar.
Bu arada, Roche çok büyük bir “ticari başarı” sağladı ve ilaç Dünya Sağlı Örgütü tarafından “temel ilaçlar” listesine alındı.
Ben de o 2009 aralık ayında yayınladığım “Tamiflu ile alâkalı ilginç gelişmeler oluyor” başlıklı makalemde o günlerde yaşananları dile getirmiştim (2).
Godlee: Hastaların hayatı tehlikede!
Godlee’ ın Profesör Bell’ e yazdığı mektubundan bazı satırlar:
“Kamu menfaati bakımından çok büyük önemi olan bu verilerin açıklanmasının reddedilmesi, Roche’ u sorumlu ilaç şirketleri grubunun dışına çıkaracaktır.
Bu verilerin açıklanması ise şirketinize ve yönetim kuruluna duyulan güvenin yenilenmesinde çok işe yarayacaktır.”
Godlee dergide yer alan makalesinde de,” www.bmj.com’ daki açık yazışmanın spesifik kişi ve kuruluşları sorumlu tutmayı amaçladığını” söylüyor ve ekliyor:
“Bağımsız araştırmacıların klinik araştırma sonuçlarına ulaşmalarının engellenmesi hastaların hayatlarını da tehlikeye atıyor.”
Profesör Bell’ e gelince. Meseleyi Roche’ a ilettiğini ve cevap beklediğini söylüyor.
Gelelim neticeye
Ben Godlee kadar umutlu değilim.
Verdiği sözü 3 sene geçmesine rağmen tutmayan Roche’ un ne Tamiflu ile ne başka ilaçlarıyla ilgili verileri açıklayacağına ihtimâl vermiyorum.
Eğer bilim dünyasından gizledikleri çalışmaların verileri Tamiflu’ nun etkili ve güvenilir bir ilaç olduğunu gösteriyor olsaydı, bunları açıklamak için bırakın kendilerine ısrar edilmesini cümle âleme davul-zurna ile duyururdu.
Vaziyet, Tamiflu’ ya ödenen milyarlarca liranın çöpe gitmiş olduğunu; bu ilacın tıp tarihi kitaplarında Thalidomide ve Vioxx’ un yanında “ayıplı ilaçlardan” biri olarak yerini alacağını gösteriyor.
KAYNAKLAR
2. https://ahmetrasimkucukusta.com/2009/12/15/yazilar/tip-yazilari/domuz-gribi/sen-neymissin-tamiflu/
***
EK 1 (19.11.2022): Cochrane reviewer sues Roche for claiming Tamiflu could slow flu pandemic
A UK epidemiologist and Cochrane Collaboration researcher is suing the drug company Roche in the US, claiming that it defrauded federal and state governments by falsely claiming that its antiviral drug oseltamivir (Tamiflu) could be a powerful tool in mitigating a flu pandemic.
Tom Jefferson, a frequent contributor to The BMJ, is suing as a private whistleblower for $1.5bn (£1.1bn; €1.4bn), roughly the amount that US public health authorities spent building up their pandemic stockpile of oseltamivir. The stockpile is still maintained today, though recent purchases have involved generic versions, as Tamiflu’s main patent expired in 2016.
Should Jefferson win, he would be awarded up to 30% of any monies recovered, while the rest would be returned to public coffers.
Jefferson’s Cochrane acute respiratory infections group was engaged by the UK government to undertake a major review of the effectiveness of oseltamivir in 2009, after an outbreak of virulent type A influenza H1N1 made headlines worldwide. The reviewers soon realised that many of their assumptions about oseltamivir rested on papers whose underlying data had never been published. They sought the detailed data of oseltamivir trials from Roche but were refused.
This led to a four year campaign—much of it conducted in the pages of The BMJ—that saw Roche and the European Medicines Agency finally release the detailed data in 2013.
Jefferson’s group studied those documents and reported in 2014.1 The first Cochrane review ever to be based on full clinical study reports rather than journal articles, it concluded that oseltamivir could reduce the duration of flu symptoms and reduce the proportion of symptomatic cases among infected people. But it did not find evidence that oseltamivir reduced disease transmission or lower respiratory complications, citing the poor design of the original studies. These are vital criteria for any pandemic treatment or prophylaxis.
Jefferson is suing Roche under the US False Claims Act, a law dating from the American Civil War, when unscrupulous contractors often sold debilitated donkeys or cartridges filled with sawdust to the US army. Roche’s actions were no different, he argues in his suit.2
Roche “originally produced Tamiflu to meet the demands of seasonal influenza, but was not satisfied with the revenue it produced,” he argues, and “embarked on a fraudulent campaign to convince the United States to add Tamiflu to its Strategic National Stockpile.”
Jefferson told The BMJ, “I’ve spent years working to uncover the truth about the efficacy and safety of Tamiflu. I myself believed what Roche said about Tamiflu’s efficacy early on. But ultimately, as alleged in the complaint, I believe Roche misrepresented that Tamiflu could stop the spread of an influenza pandemic—when the Roche generated evidence doesn’t show the drug can even stop viral transmission, let alone prevent complications or deaths related to influenza.
“Tamiflu can cause significant adverse effects in patients. I brought this suit because I felt so many, including the US government, have been misled by what I allege in the complaint are false statements about Tamiflu’s efficacy for pandemic use.”
A Roche spokesman commented, “The United States Department of Justice (DOJ) filed notice in the district court that DOJ was declining to intervene and take over the litigation following its investigation of the allegations in the complaint. Roche fully cooperated with DOJ’s investigation. Roche has complete confidence in the safety and efficacy of Tamiflu. The company plans to vigorously defend itself against these allegations if Thomas Jefferson decides to pursue the litigation.”
The US government intervenes to take over as plaintiff in about a quarter of whistleblower lawsuits brought under the False Claims Act. But the whistleblower can still recover money for the government even when it does not intervene.
Jefferson’s attorney Clayton Halunen said, “The government’s decision to intervene or not intervene in any given False Claims Act case does not speak to the merits of the case. To insinuate otherwise misrepresents the state of the law. This lawsuit does not focus on the claimed safety or efficacy of Tamiflu when used in treatment of seasonal influenza. It alleges that Roche sold the United States a bill of goods on Tamiflu’s efficacy for use in an influenza pandemic. We fully expect Roche to vigorously defend itself—it has a billion dollar stockpile sale to protect. We are confident in the strength of our case and look forward to deposing Roche executives about the alleged scheme.”
Kaynak: https://www.bmj.com/content/368/bmj.m314
Makale: Cochrane reviewer sues Roche for claiming Tamiflu could slow flu pandemic
***
EK 2 (2.8.2023): Story of Influenza Antivirals: Part 27
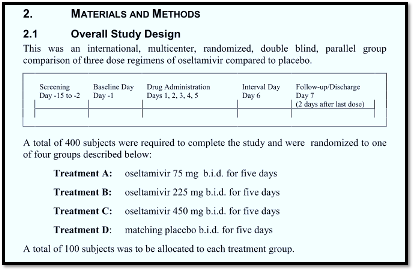
This is the latest in a series of posts telling the story of the discovery of unseen trials on the anti-influenza drugs Tamiflu and Relenza, published in Trust the Evidence and cross-posted in Maryanne Demasi reports. This post contains further reflections on the glib word “placebo”, frequently used in biomedical literature without further examination or thought. In the antivirals story, we got to the point when the European regulator (EMA) released the clinical study reports (CSRs) of 15 Tamiflu (Oseltamivir) trials in late 2011. This was followed by the first version of our Cochrane review and fed intense media coverage of the story. Of the 15 Oseltamivir trial CSRs held by EMA, only one was complete. The trial, known as WP16263, consisted of 8545 pages, more than 1000‐fold greater than its published version (Dutkowski 2010), which consists of 7 pages. The trial had been carried out on healthy volunteers to assess any cardiotoxic properties of Tamiflu. None of us had ever read that many pages describing a single clinical trial, but it turned out that most of the pages were printouts of “listings”, i.e. individual participant data. Methods and results came to about 500 pages. The 8-page summary helpfully showed us what went on: Four hundred adult volunteers were randomly assigned to four arms (100 in each arm), and the study described itself as a multicentre, double-blind randomised design. By sheer chance, our tired eyes fell on a document called the Certificate of Analysis, which is not always present in regulatory data. (Graphics courtesy of Peter Doshi) A Certificate of Analysis is an authenticated document certifying the constituents, quality, colour and purity of a product. In this example, we have a record of the appearance of Tamiflu and placebo capsules. The Tamiflu capsule had a light yellow cap, but the placebo had an ivory-coloured cap. The placebo contained dehydrocholic acid to simulate the bitter taste of the active compound. So the content, taste and aspect of a placebo is important. We do not know whether the different coloured capsules affected the results of the trial, but it is possible. Calling it a “double-blinded” study however, was not the correct description. For GSK’s Relenza, what appeared as mere details, such as the content of placebo capsules, became prominent when we discovered that the placebo blister of Zanamivir used in the trial contained lactose powder. If you are sensitive to lactose, you are likely to get an asthma-type reaction. Notably, Reker et al published a list of critical ‘inactive’ ingredients that are added to placebos which may act as allergens including lactose, polyethylene glycol (PEG), corn starch, povidone or dyes such as Brilliant Blue. GSK justified this inclusion by saying that lactose was an excipient of the active principle. Hence, the placebo blisters were only minus the active principle, as any credible placebo should be. Despite this, our meta-analysis showed a significant increase in the risk of asthma episodes in recipients of the active principle. Thankfully, in this case, FDA reviewers picked this up. It’s not often disclosed by manufacturers or publications. A recent analysis of 113 placebo-controlled RCTs published in high-impact medical journals in the year 2016 found that a minority (19.5%) of them reported the contents of placebos. The aim of blinding is to reduce bias due to the knowledge of which intervention or control is being received by study participants. Blinding in a trial can be single, double-blind or triple-blind; however, what is important is defining who was blinded, as the terms are often easily confused. An analysis of 250 randomised trials reported in 33 meta-analyses showed that studies that did not report double-blinding showed average odds ratios that were 17% higher than studies that did not. A lack of double blinding was associated with a greater exaggeration of intervention effect estimates in trials with subjective outcomes (i.e. symptoms) compared to objective outcomes, such as death, where the lack of blinding had a negligible effect. The bottom line appears to be that often the term “placebo”, taken at face value, may conceal differences which could affect study results. And take any trial published in biomedical journals with a pinch of salt. References Jefferson T, Jones MA, Doshi P, Del Mar CB, Heneghan CJ, Hama R, Thompson MJ. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database of Systematic Reviews 2012, Issue 1. Art. No.: CD008965. DOI: 10.1002/14651858.CD008965.pub3. Accessed 19 July 2023. Dutkowski R, Smith JR, Davies BE. Safety and pharmacokinetics of oseltamivir at standard and high dosages. International Journal of Antimicrobial Agents 2010;35:461–467. Golomb BA, Erickson LC, Koperski S, Sack D, Enkin M, Howick J. What’s in placebos: who knows? Analysis of randomized, controlled trials. Ann Intern Med. 2010 Oct 19;153(8):532-5. doi: 10.7326/0003-4819-153-8-201010190-00010. PMID: 20956710. Webster RK, Howick J, Hoffmann T, Macdonald H, Collins GS, Rees JL, Napadow V, Madigan C, Price A, Lamb SE, Bishop FL, Bokelmann K, Papanikitas A, Roberts N, Evers AWM. Inadequate description of placebo and sham controls in a systematic review of recent trials. Eur J Clin Invest. 2019 Nov;49(11):e13169. doi: 10.1111/eci.13169. Epub 2019 Sep 30. PMID: 31519047; PMCID: PMC6819221. Howick J, Webster RK, Rees JL, Turner R, Macdonald H, Price A, Evers AWM, Bishop F, Collins GS, Bokelmann K, Hopewell S, Knottnerus A, Lamb S, Madigan C, Napadow V, Papanikitas AN, Hoffmann T. TIDieR-Placebo: A guide and checklist for reporting placebo and sham controls. PLoS Med. 2020 Sep 21;17(9):e1003294. doi: 10.1371/journal.pmed.1003294. PMID: 32956344; PMCID: PMC7505446. |
***

















Tamiflu harcamaları çöpe gitmedi yapıp satanların cebine gitti. Zaten amaç da o idi.